
IRIS SARS COV-2 IgM /
IgG ANTI-BODY TEST KIT



This is a rapid test for the qualitative detection of IgM and IgG antibodies against SARS-CoV-2 in human blood (collected from a vein or fingertip), serum or plasma. The test is for in vitro diagnostic use only and for professional use only.
MORE
IRIS SARS CoV-2 IgG IgM Rapid Test
Technical Report and Clinical Data Analysis
1.0 Introduction
IRIS SARS CoV-2 IgG IgM Rapid Test Technical Report and Clinical Data Analysis
The IRIS SARS CoV-2 IgG IgM is a colloidal gold-based immunochromatographic strip assay produced by Alpha Pharma Industry (Bari, Italy). The test is rapid (10-14 min) and is based on a lateral flow immunoassay targeted to evaluate the presence or absence of anti-SARS-CoV-2-IgM and anti-SARS- CoV-2-IgG. The test is validated for different human specimens: capillary blood, venous whole blood, serum and/or plasma.
The IRIS SARS CoV-2 IgG IgM utilizes Anti-human IgG and anti IgM against the Receptor-Binding Domain of the COVID-19 spike protein recombinant antigen. The strip test consists by a nitrocellulose membrane incorporating mouse anti-human-IgM and IgG monoclonal antibody, plus anti-rabbit-IgG immobilized in different position (M and G lines) and in control line (C line), respectively.
The use of the test is extremely simple and rapid: the sample (10-15 ul) is deposited in sample-port and few second later 2 drops are added (the buffer is supplied together with the test in a dropper bottle). The blood macromolecules are moved forward by capillarity and "walk" along the nitrocellulose strip which has been absorbed by a mixture of recombinant antigen AuNP-COVID-19 and AuNP-rabbit-IgG. If anti-SARS CoV-2 IgM antibodies are present in the patient's blood, they bind to the viral antigen labelled with colloidal gold, then forming a sandwich with the anti-human IgM monoclonal antibody (present only on one line marked by the letter M); in this case the line will turn pink-red. Similarly, for IgG, in a second line in which monoclonal antibodies to human IgG are present.
If both lines are not dyeing, the test will be considered as negative; vice versa the development of a coloured band (even if not very intense) on the IgG and/or IgM line indicates the presence of anticovid antibody in the patient blood and the test will be considered positive. The device also contains a quality control line C, which must always colour and check that the kit is working properly.
2.0 Experimental data results
2.1.1 Preliminary test on sensitivity and specificity
Test are performed in the clinical setting of Catanzaro Lido Polyclinic (Dr Maurizio Cipolla, MD, Pathologist) in cooperation with the Catanzaro Hospital (Dept of Infectious Diseases) for the RT-PCR execution according with the Italian Government Guide Lines.
Sample size 15 adults of both sexes, aged 30-70 yr., with suspected diagnosis of Covid19 infection.
Operating Procedures: IRIS SARS CoV-2 IgG IgM test execution in the same day of pharyngeal swab for RT-PCR detection of Covid-19 according with WHO guideline and procedure advised by Regione Calabria and Italian Health Ministry. IRIS SARS CoV-2 IgG IgM test samples: capillary blood Statistics: non parametric test (Cohen’s K and exact McNemat test). Probability of Agreement, Concordance, and Cohen’s K categorization according to DG Altman and R. Kwiecien1
The Results for IgG test: Cohen's k = 1.000; exact McNemar test: p > 0.999. Sensitivity 100,0% (95% Intervals of Confidence= 39.7-100.0). Specificity 100,0% (95%IC= 71,5-100.0).
Results for IgM test Cohen's k = 0.842; exact McNemar test: p > 0.99. Sensitivity 100,0% (95% Intervals of Confidence= 39.7-100.0). Specificity 90,1% (95%IC= 58.7-99.7).
2.1.2 Final test on sensitivity and specificity
The tests were performed as previously described (see 2.1.1). The overall sample (pooled data) consists of 161 adult subjects of both sexes
2.1.2.1 IgM results: Cohen's k = 0.963, McNemar exact test: p > 0.9999.
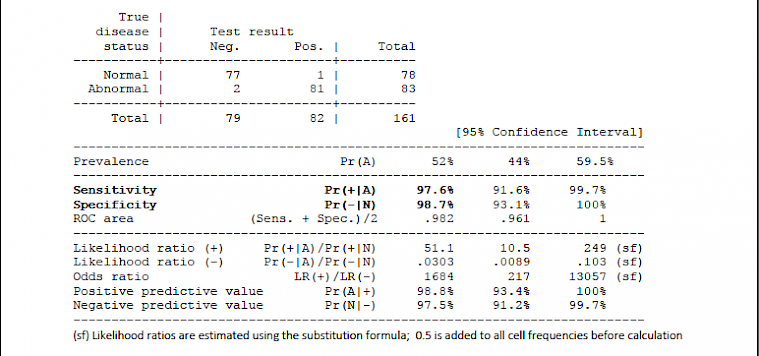
2.1.2.2 IgG results: Cohen's k = 0.963, McNemar exact test: p = 0.2500
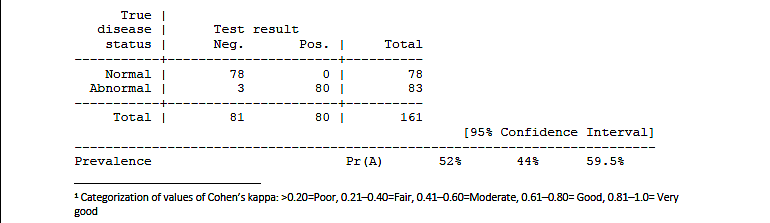
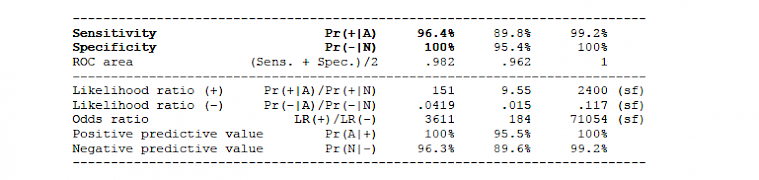
2.2.1 Clinical protocol (in- and out-patients)
Test are performed in the clinical setting of San Camillo Forlanini Hospital in Rome (Dr Gabriella Parisi) (Dept of Microbiology and Virology). The PCR test are performed according with Italian Istituto Superiore di Sanità (ISS) and Italian Health Ministry guideline and recommendations
Sample size 365 patients selected on the basis of the symptoms or candidate to surgery, from the Emergency, Surgery and Internal Medicine Departments. Patients of both sexes (no age-based exclusion criteria) with suspected diagnosis of Covid19 infection or candidate to the PCR before admission to surgery.
Operating Procedures: IRIS SARS CoV-2 IgG IgM test execution within 36-48 hrs before or after pharyngeal swab for RT-PCR detection of Covid-19 according with WHO guideline and procedure advised by ISS and Italian Health Ministry. IRIS SARS CoV-2 IgG IgM test samples: venous whole blood or plasma after REB separation by centrifugation.
Results:
On 365 patients, 353 showed the same results both with PCR and IRIS SARS CoV-2 test. The concordance rate resulted of 97%. In 12 patients with discordant results, 7 resulted negative to the RT-PCR and positive for IgM only (n=6), for IgM and IgG (n=1<) while the RT-PCR resulted negative (see footnote 1, previous page). After a second oropharyngeal swab for RT-PCR second control performed on discordant patients, 5 patients tested positive to RT-PCR, thus the used test gave a 98.1% (358/365) concordance (e.g., accuracy), with an expected CI95% from 96.1% to 99.2%
2.2.2 Healthcare personnel survey
Test are performed on the Doctors and Nurses of the San Camillo Forlanini Hospital in Rome (Dr Gabriella Parisi, Dept of Microbiology and Virology). The PCR test are performed as previously described.
Sample size 180 doctors and nurses selected among the San Camillo-Forlanini Hospital healthcare personnel (adults, both sexes) on the basis of risk exposition to Covid-19 infection.
Operating Procedures: IRIS SARS CoV-2 IgG IgM test execution together with the pharyngeal swab for RT-PCR detection of Covid-19, according with WHO guideline and procedure advised by ISS and Italian Health Ministry. IRIS SARS CoV-2 IgG IgM test samples: venous whole blood or plasma and/or capillary blood.
Results: concordant results were documented in 176/180 hospital employees, the accuracy was 97.8% (176/180), with an expected CI95% from 94.4% to 99.4%. Discordant results (n=4): 1 false negative results -for both IgM and IgG rapid test- with positive oropharyngeal swab; 2 false negative of RT-PCR; one was classified as "doubtful IgM positivity" (due to the difficulty of reading the IgM band): this patient had a positive PCR test and certainly negative IgG band and should be included among the possible false negatives of the rapid test.
*** * ***
The test carried on the whole San Camillo sample (patients plus health workers) gave a 97.8% accuracy, with an expected CI95% ranging from 96.2% to 98.9%.
2.3 IRIS SARS CoV2 test in the setting of the occupational medicine and industry worker protection
Tests were carried out on the employees of a company (Sorical SPA, General manager Pia Chiarella) working in the field of drinking water and water supplies of almost all the Calabrian municipalities. The aim is checking all employees of the industry (continuous monitoring of Covid-19 infection by rapid test and quarantine any COVID-19 positive employee).
Operating Procedures: all employees were invited to take the IRIS SARS CoV-2 IgG IgM rapid test during working hours, inside the factory. The test was repeated every 15 days. Employees were also given a computerized Covid card (with information on their health, co-morbidity and any symptoms present) and the data were sent to the competent doctor. The RC-PCR test was performed only if the rapid test was positive.
Sample size: 172 healthy employees (aged 20-70, males and females) were tested. At present two controls were performed (total 344 IRIS SARS CoV-2 tests).
Results: only 1 case resulted positive to the IgM test (IgG negative, first RT-PCR test negative): the employee was released from the work (quarantine) waiting the second RT-PCR test in accordance with the Italian legal provisions and with the guidelines. No new cases of seroconversion were detected during the first phase of the follow up.
3.0 Conclusions
The IRIS SARS-CoV-2 IgG / IgM rapid test has a very high sensitivity and specificity; has been successfully used in different settings, for monitoring and diagnosing patients (belonging to General Medicine, Emergency Dept or Internal Medicine) and for the periodic survey of industrial and healthcare personnel.
We consider this test valid and reliable in several sectors (food industry, hospitals and medical centres, other strategic industries) both for the high sensitivity and specificity (and therefore accuracy) and for the extreme ease of use and reading. In fact, over 900 tests carried out during the study and validation phase in Italy, no cases of difficulty / impossibility of execution were reported, even in non-specialist environments; in only one case was a doubt reported on reading the coloured band.
In our opinion, a test with such high sensitivity and specificity is also indicated for epidemiological purposes on large sections of the population (general population and/or high-risk groups). From a clinical point of view, it is indicated for the diagnosis of Covid19 infection and / or the differential diagnosis with respect to other diseases, according to good clinical practices, which require the doctor to consider all the diagnostic elements (clinic, instrumental, laboratory, included the RT- PCR). Furthermore, the use of the IRIS SARS CoV-2 test can reveal patients with false negative RT- PCR, as suggested by the WHO guidelines and the literature. It should be noted that the execution of the rapid serological test, while not replacing the use of swabs to identify viral RNA, is not operator-dependent and therefore very suitable for the setting of primary care2.
Coordinators of the Scientific Committee
DR. Maurizio Cipolla MD, Pathologist
President of Digital Sit Calabria (Italian Society of Digital Health and Telemedicine Scientific director of the multicronicity plans Regione Calabria (Italy)
Scientific director of Digitcal Srl
Prof. Antonio Vittorino Gaddi, MD, PhD
Research Doctor in Experimental Medicine and Atherosclerosis - Consultant: Cardiology, Geriatrics and Gerontology
Main Investigator of Health Research and Development SrL, IT -Scientific Director of Qi International Ltd, London, UK
Past President of the Medicine and Surgery undergraduate course of the Bologna University -
Past Director, of Giancarlo Descovich Bologna Center of Atherosclerosis and Metabolic Diseases
President of Digital SIT-EMR (Italian Society of Digital Health and Telemedicine, Emilia Romagna Region), Director of Scientific Board of EuroGenLab (BO) and Health-Lab, GT Foundation (MO).
Lugo Medica and Caravelli Lab & EuroGenLab, via Acquacalda 3 (Lugo, RA) and via Zamboni 8, Bologna,051 231531 -mobile+39 3341953354 https://www.researchgate.net/profile/Antonio_Gaddi - https://it.wikipedia.org/wiki/Antonio_Vittorino_Gaddi
4.0 References
B. B. Practice, “Coronavirus disease 2019- Situation report 76,” World Heal. Organ., vol. 2019, no. April, p. 2633, 2020, doi: 10.1001/jama.2020.2633.
B. Meyer, C. Drosten, and M. A. Müller, “Serological assays for emerging coronaviruses: Challenges and pitfalls,” Virus Res., 2014, doi: 10.1016/j.virusres.2014.03.018.
C. G. B. Caraguel, H. Stryhn, N. Gagné, I. R. Dohoo, and K. L. Hammell, “Selection of a cutoff value for real-time polymerase chain reaction results to fit a diagnostic purpose: Analytical and epidemiologic approaches,” Journal of Veterinary Diagnostic Investigation. 2011, doi: 10.1177/104063871102300102.
C. Sheridan, “Fast, portable tests come online to curb coronavirus pandemic,” Nat. Biotechnol., 2020, doi: 10.1038/d41587-020-00010-2.
D. Lin et al., “Evaluations of serological test in the diagnosis of 2019 novel coronavirus (SARS-CoV-2) infections during the COVID-19 outbreak,” medRxiv, 2020, doi: 10.1101/2020.03.27.20045153.
DG Altman. Practical statistics for medical research. 1st edition. Oxford: Chapman and Hall. 1991:1– 611.
G. Lippi, A.-M. Simundic, and M. Plebani, “Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19),” Clin. Chem. Lab. Med., 2020, doi: 10.1515/cclm-2020-0285.
J. P. T. Higgins, S. G. Thompson, J. J. Deeks, and D. G. Altman, “Measuring inconsistency in meta- analyses,” British Medical Journal. 2003, doi: 10.1136/bmj.327.7414.557.
J. Xiang et al., “Evaluation of Enzyme-Linked Immunoassay and Colloidal Gold- Immunochromatographic Assay Kit for Detection of Novel Coronavirus (SARS-Cov-2) Causing an Outbreak of Pneumonia (COVID-19),” medRxiv, 2020, doi: 10.1101/2020.02.27.20028787.
J. Zhang et al., “Serological detection of 2019-nCoV respond to the epidemic: A useful complement to nucleic acid testing,” medRxiv, 2020, doi: 10.1101/2020.03.04.20030916.
J. Zhao et al., “Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019,” medRxiv, 2020, doi: 10.1101/2020.03.02.20030189.
L. Lan et al., “Positive RT-PCR Test Results in Patients Recovered From COVID-19.,” JAMA, 2020, doi: 10.1001/jama.2020.2783.
L. Liu, W. Liu, S. Wang, and S. Zheng, “A preliminary study on serological assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 238 admitted hospital patients,” medRxiv, 2020, doi: 10.1101/2020.03.06.20031856.
M. El-Tholoth, H. H. Bau, and J. Song, “A Single and Two-Stage, Closed-Tube, Molecular Test for the 2019 Novel Coronavirus (COVID-19) at Home, Clinic, and Points of Entry,” 2020, doi: 10.26434/CHEMRXIV.11860137.V1.
P. Winichakoon et al., “Negative Nasopharyngeal and Oropharyngeal Swab Does Not Rule Out COVID-19.,” J. Clin. Microbiol., 2020, doi: 10.1128/JCM.00297-20.
P. Zhang et al., “Evaluation of recombinant nucleocapsid and spike proteins for serological diagnosis of novel coronavirus disease 2019 (COVID-19),” medRxiv, 2020, doi: 10.1101/2020.03.17.20036954.
Q. Li et al., “Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia,” N. Engl. J. Med., pp. 1199–1207, 2020, doi: 10.1056/nejmoa2001316.
Q. Y. Gao, Y. X. Chen, and J. Y. Fang, “2019 Novel coronavirus infection and gastrointestinal tract,” Journal of Digestive Diseases. 2020, doi: 10.1111/1751-2980.12851.
R. Porcheddu, C. Serra, D. Kelvin, N. Kelvin, and S. Rubino, “Similarity in Case Fatality Rates (CFR) of COVID-19/SARS-COV-2 in Italy and China,” J. Infect. Dev. Ctries., 2020, doi: 10.3855/jidc.12600.
R. Kwiecien, A. Kopp-Schneider, M. Blettner: Concordance Analysis, Dtsch Arztebl Int. 2011 Jul; 108(30): 515–521. doi: 10.3238/arztebl.2011.0515
S. L. Bai et al., “[Analysis of the first cluster of cases in a family of novel coronavirus pneumonia in Gansu Province].,” Zhonghua Yu Fang Yi Xue Za Zhi, 2020, doi: 10.3760/cma.j.issn.0253- 9624.2020.0005.
V. M. Corman et al., “Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR,” Euro Surveill., 2020, doi: 10.2807/1560-7917.ES.2020.25.3.2000045.
W. Wang et al., “Detection of SARS-CoV-2 in Different Types of Clinical Specimens,” JAMA, 2020, doi: 10.1001/jama.2020.3786.
WHO, “Population-based age-stratified seroepidemiological investigation protocol for COVID-19 virus infection,” no. March, pp. 1–19, 2020.
World Health Organization, “Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases,” vol. 2019, no. January, pp. 1–7, 2020.
World Health Organization, “WHO Director-General’s opening remarks at the mission briefing on COVID-19,” https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at- the-media-briefing-on-covid-19---11-march-2020, 2020. .
Z. Li et al., “Development and Clinical Application of A Rapid IgM-IgG Combined Antibody Test for SARS-CoV-2 Infection Diagnosis.,” J. Med. Virol., 2020, doi: 10.1002/jmv.25727.
1 Categorization of values of Cohen’s kappa: >0.20=Poor, 0.21–0.40=Fair, 0.41–0.60=Moderate, 0.61–0.80= Good, 0.81–1.0= Very good
2 Gaddi AV et al: “The strategic alliance between Clinical and Molecular Science in the war against SARS-CoV-2, with the rapid- diagnostics test as an indispensable weapon for front line doctors”. Position paper signed by 28 Health and Research Italian Centers, in submission to Int J Mol Sci.
IRIS SARS COV 2
"Iris Sars Cov 2"
The Iris Sars Cov-2 IgM / IgG Antibody Test Kit is a rapid test for the qualitative detection of IgM and IgG antibodies against SARS-CoV-2 in human blood (collected from a vein or fingertip), serum or plasma. The test is for in vitro diagnostic use only and for professional use only. It is intended for clinical laboratories and for use by healthcare professionals only for tests performed in treatment centers. It is not intended for home testing.
The Iris Sars Cov-2 IgM / IgG antibody test kit is based on immunoassay technology. The kit contains: 1) Conjugate buffer: recombinant SARS-CoV-2 antigen conjugated to colloidal gold bound to FITC, FITC antibodies and gold labeled quality control antibodies. 2) Nitrocellulose membrane: equipped with two detection bands (band for IgG and band for IgM) and one quality control band (band C). The IgM band coated with monoclonal anti-human rat IgM antibodies detects IgM anti-SARS CoV-2 antibodies. The IgG band coated with anti-human-rat monoclonal IgG antibodies detects IgG anti-SARS CoV-2 antibodies. Band C is coated with antibodies for quality control.
When the blood/serum/plasma sample is added to the well of the device, it reacts with the reagents in the device. If the specimen entered contains IgM antibodies, it will bind to the viral antigen labeled with colloidal gold to form a sandwich complex with the anti-human IgM monoclonal antibody coated on the IgM band. The IgM band appears purple, indicating that the SARS CoV-2 IgM antibody is positive. If the entered sample contains IgG antibodies, it will bind to the viral antigen labeled with colloidal gold and form a sandwich complex with the anti-human IgG monoclonal antibody coated on the IgG band. The IgG band appears purple, indicating that the SARS CoV-2 IgG antibody is positive.
If none of the IgG or IgM bands stains, the test result is negative. The test also contains the C band for quality control, which appears purple-red if the test is valid. If the C band for quality control does not appear: The test is not valid even if the bands for IgM and/or IgG detection should appear
KIT-COMPOSITION
Each box contains 40 kits.
Each kit contains: the device, the buffer, the pipette (optional) and the package insert.
Materials required but possibly not included: Sterile lancing device (for blood from fingertips), alcohol swab, clock / timer.
STORAGE AND RECOMMENDATIONS FOR USE
• Store the kit in a cool, dry place at a temperature between 2 ° and 30 ° C. Keep it away from direct sunlight. Exposure to temperatures and/or humidity other than those specified may cause erroneous results.
• Do not freeze or cool. Use the kit at a temperature between 15 ° and 30 ° C.
• Use the kit at a humidity between 10% and 90%.
• Do not use kits that are past their expiration date (printed on the aluminum test bag and on the packaging).
Note: All expiration dates are written in the format YYYY-MM Year-Month. For example, 2021-06 means June 2021.
WARNINGS, PRECAUTIONS AND RESTRICTIONS OF USE
• The results of IgM / IgG antibodies obtained from the test should NOT be used as the sole basis for diagnosis or to rule out SARS-CoV-2 infection or to provide information on infection status.
• Negative results do not exclude SARS-CoV-2 infection, especially in individuals who have been exposed to the virus. In order to exclude infection in persons with a positive rapid test, follow-up tests with molecular diagnostics and/or CT should be considered.
• Positive results may be due to past or present infection with non-SARS CoV-2 coronavirus strains such as coronavirus HKU1, NL63, OC43 or 229E. Follow-up testing with molecular diagnostics and/or CT should be considered to confirm the rapid test result.
• This rapid test is not intended for home use.
• This rapid test is not intended for screening of donated blood.
• Do not use highly hemolytic samples.
• Further molecular diagnostics and/or CT is recommended to determine the actual physical situation.
• Do not use the same test again. Do not use the kit after the expiration date.
• Use only whole human blood (from a vein or fingertip), serum or plasma as specimen. Follow the package insert for accurate results.
All parts of the kit are considered biologically hazardous and may possibly transmit infectious diseases by bloodborne pathogens even after cleaning and disinfection. Take appropriate precautions and follow all applicable regulations when disposing of used test kits.
PROCEDURE FOR PERFORMING THE TEST
Balance the kit and buffer at a temperature between 15° and 30° C before starting the test.
Place the device on a clean, flat surface.
Insert 10 μl whole blood (collected from a vein or fingertip), serum or plasma into the well of the device.
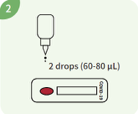
Add 2 drops (approx. 60-80 μl) of buffer into the well of the device
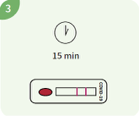
Read the test result in the 15th minute. Read the result no later than 20 minutes after testing.
Note: Handle the buffer carefully and avoid any contact with eyes or skin. In case of contact with eyes or skin: wash thoroughly with water.
INTERPRETATION OF THE RESULTS
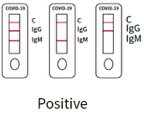 1. positive result
1. positive result
SARS-CoV-2 IgM antibody positive:
Quality Control C band and IgM band both stain and appear, while the IgG band does not stain and does not appear.
SARS-CoV-2 IgG antibody positive:
Quality Control C and IgG bands are stained and appear while the IgM band is not stained and does not appear.
SARS-CoV-2 IgM and IgG antibodies positive:
All 3 bands appear, the C of the quality control and those of IgG and IgM.
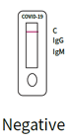 2. negative result:
2. negative result:
Only the C band of the quality control is stained and appears without other lines for detection of IgM or IgG. This indicates that the test result is negative for both IgM antibodies and SARS-CoV-2-IgG.
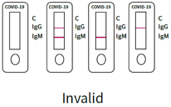 3. invalid result:
3. invalid result:
The quality control C band is not displayed to indicate that the test is invalid, whether or not the IgG and IgM bands are stained. You must take a new sample and perform another test with a new kit.
QUALITY CONTROL
InterInternal procedural tests are included in the test. A colored band that appears in the control region (C) is an internal procedural control and confirms that the sample volume is adequate and that the proper procedural technique is being used. Control standards are not supplied with this kit. However, it is recommended that the positive and negative controls be tested as good laboratory practice to confirm the test procedure and to verify the performance of the test.
CONTACT
DOJA T∙E∙C
Vertriebs- und Beratungsgesellschaft mbH
Schillerstraße 20
72144 Dusslingen / Germany
Phone: +49 7072 9293 0
Fax: +49 7072 9293 33
E-Mail:
